M S Nurul Aniyyah1*, Z Idhamnulhadi1, A A Azharin Shah1, H Lili Shakirah1, A Suhaila1, H Norazlina1, M Hajaratul Najwa1,
1Faculty of Engineering Tecnologu,University College TATI, Jalan Panchur,Teluk Kalong, 24000, Kemaman, Malaysia, Email: aniyyah@uctati.edu.my
Abstract.
The use of harmful alcohol-based disinfectants and sanitizers was a major concern throughout the CoVID-19 era. Frequent use of alcohol-based sanitizer can dry up the skin, and the effect is worsening for individuals with sensitive skin. Alcohol-based disinfectants are flammable and can ignite if used near a flame, spark, or other source of ignition. Using the electrolysis of sodium chloride (NaCl) aqueous solution method, this study aims to make Hypochlorous Acid (HOCl), a safe disinfectant and sanitizer. Two critical parameters were tested on the electrolysis effect of producing HOCl. The first is the amount of sodium chloride (NaCl) present, while the second is the type of electrode used, which are carbon, graphite, and titanium. The results showed that 10 grams of NaCl produces 50-200 ppm of HOCl, which is good for sanitizing purposes, and 30 grams of NaCl produces 500-800 ppm of HOCl, which is good for disinfecting purposes. The graphite electrode was also demonstrated to be capable of producing a clean HOCl solution. Using a UV-vis spectrophotometer, the effectiveness of the HOCl produced was determined, and it was discovered that HOCl is capable of killing bacteria. As a result, HOCl can be applied as a safe disinfectant and sanitizer in the fight against COVID-19.
More than a year has passed since the World Health Organization (WHO) declared coronavirus disease 2019 as a pandemic. The need for disinfectants and sanitizers has increased globally, particularly in the medical and health care sectors, as well as in other industries and for personal usage. Sanitizing reduces the numbers of germs, whereas disinfection eliminates germs by applying an EPA-registered microorganism-killing solution [1]. One of the most serious problems is the use of hazardous alcohol
based disinfectants and sanitizers. Frequent use of alcohol-based sanitizers can dry up the skin, and the effect is worsening for individuals with sensitive skin. Alcohol-based disinfectants are flammable and can ignite if used near a flame, spark, or other source of ignition. It is important to find safe and effective disinfectants and sanitizers like Hypochlorous Acid (HOCl) to combat COVID-19.
EW is an oxyacid of chlorine containing monovalent chlorine that serves as an oxidizing or reducing agent. Hypochlorous acid, commonly known as electrolyzed water, is an oxyacid of chlorine containing monovalent chlorine that acts as an oxidizing or reducing agent. It’s a weak acid that can only exist in solution because it’s exceedingly unstable. In oxidation-reduction reactions, where electrons are transported from one molecule to another, the molecules are electron–accepting. EW is a product generated by electrolysis of a dilute NaCl solution in an electrolysis cell with a semi-permeable membrane that physically separates the anode and cathode while allowing specific ions to pass through [2,3]. HOCl is the primary antibacterial agent in sanitizers and disinfectants, and it is approved for use against SARS-CoV-2 by the US Environmental Protection Agency (EPA) under regulation [4]. Alkaline EW, acidic EW, and neutralized EW are the three different types of EW that can be produced. However, acidic EW, as illustrated in Figure 1 for the process diagram [3,5], is the most used and studied type. The electrode voltage is usually set between 9 and 10 volts [6]. Sodium chloride (NaCl) dissolved in deionized water (brine) breaks down into negatively charged chloride (Cl-) and positively charged sodium (Na+) during electrolysis. Hydroxide (OH-) and hydrogen (H+) ions are generated at the same time. Ions with a negative charge, such as Cl- and OH-, lose electrons at the anode, forming oxygen gas (O2), chlorine gas (Cl2), hypochlorite ion (OCl-), hypochlorous acid (HOCl), and hydrochloric acid (HCl). Meanwhile, ions with a positive charge, H+ and Na+ travel to the cathode to generate hydrogen gas (H2) and sodium
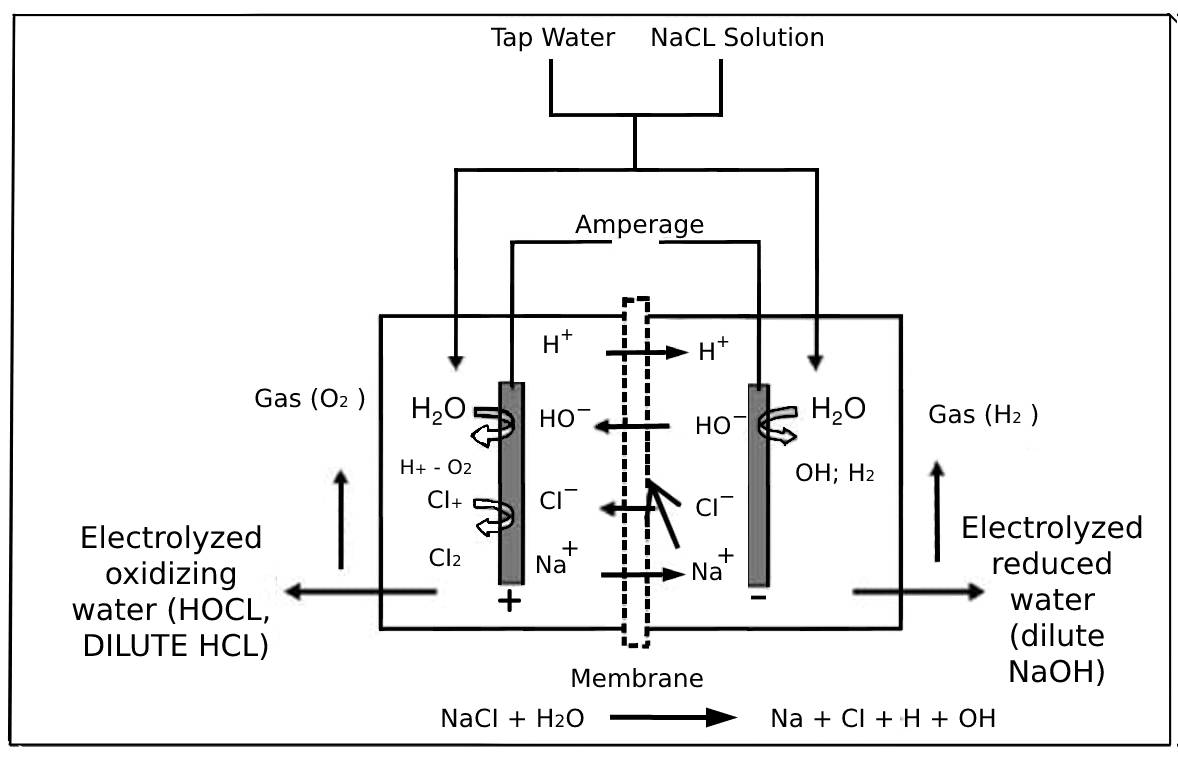
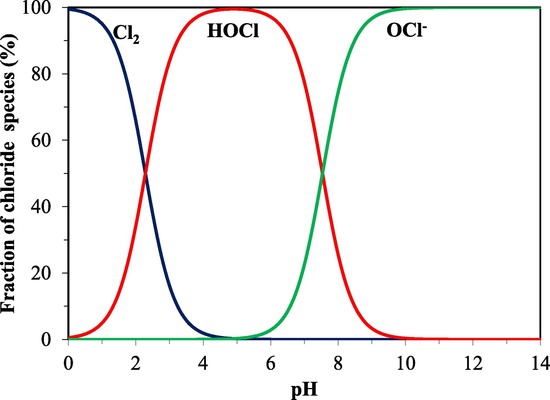
The pH of a solution determines the amount of HOCl and hypochlorite ion (OCl). Between pH 5 and 7, HOCl is the most predominant species found. The concentration of HOCl is optimum and dissociation is minimal within this pH range. Figure 2 shows that at higher pH, OCl- is generated, but at lower pH, the solution is a mixture of chlorine (Cl2) and HOCl in solution. On the market, there are various EW producing instruments that can be divided into two types. The first type is an electrolyzer with a membrane that produces acidic and basic EW, known as two cell chambers, whereas the second type is an electrolyzer without a membrane that produces neutral EW, known as single cell chambers [5]. The physical properties and chemical composition of hypochlorous acid produced vary depending on the content of NaCl solution, amperage level, time of electrolysis, temperature, and pH [7,8]. EW has received much interest as a chlorine-free alternative to other disinfectants and sanitizers. Acidic EW has received the most attention because of its highly effective antibacterial activity as a main agent, which may penetrate cells and oxidize important metabolic components [9]. EW is identified as the best way to save money on chemicals and is considered environmentally friendly because it simply uses tap water and NaCl [10]. Furthermore, treating or disposing of the chemicals will only have a negative impact on the environment and increase operating costs. Because EW may be generated on site and does not require any storage space, it is seen as a potential solution to the storage problem [11]. Moreover, a century has passed since the first discovery of EW [5]. EW was first used in agriculture in the 1950s. The use of EW in drinking water disinfection and wastewater treatment plants was proposed in the late 1970s and early 1980s [1]. EW was also developed in Russia, where it was utilized in medical institutes for water decontamination, water regeneration, and disinfection [2]. After that, in the 1980s, EW was commercially introduced in Japan’s food sector as sanitation water and an automatic dispenser in the food processing and soda industries. Since then, EW has caught the interest of many researchers, and various publications on its effectiveness as a disinfectant in the food sector have been discovered [12]. Concerns regarding foodborne pathogens identified on raw vegetables in the late 1990s prompted researchers to investigate and explore alternate sanitizers such as EW. With recent developments in technology and the availability of better equipment, EW has benefited from the electrolysis of NaCl aqueous solution.
HOCl production and application have been the focus of many researchers. HOCl has been utilized in the electrolysis process to generate chlorine-based antiseptics by mixing drinking water with small amounts of sodium hydroxide at various concentrations. According to the findings, 0.005-0.01 percent HOCl water may destroy most bacteria and pathogens in 12 seconds [13]. The ability of neutral EW as a sanitizer to reduce biofilms of Escherichia coli O157:H7, Salmonella enteritidis, and Listeria monocytogenes on the surface of tomato, plastic, and wooden cutting boards has been demonstrated [14,15]. Red radish seedlings infected with Listeria monocytogenes were treated with EW at a dosage of 60 to 80 ppm, and the germination rate increased from 93.5 percent to 97.7% [16]. After soaking fruit in an EW water solution for as little as 10 minutes, fruit rot caused by the fungus Botryosphaeria berengeriana was suppressed in pears [17]. EW contains antifungal properties and can lower Candida albicans biofilm levels on denture resins: a denture storage and disinfection device [18]. EW has lately been recommended as the disinfectant of choice in an oral and maxillofacial surgery office [19] based on a recent study.
The effect of various electrolyzing factors on the formation of HOCl has been described in the literature [20], including electrode types, electrode conductivity, NaCl concentration, salt type, flowrate, temperature, electrical potentials, and electrolysis time. Due to its corrosion-resistant properties of stainless steel, brass, and aluminium as anodes in water electrolysis, many studies on electrode type have been conducted. Unfortunately, when stainless steel corrodes, hazardous chemicals are released, brass deposits copper on the cathode, speeding up the rusting of steel, and aluminium corrodes quickly. Because of their inexpensive cost, higher electrical and thermal conductivity, and relative inertness in alkaline solution compared to metals, carbon-based electrodes such as carbon and graphite are far more practical and attractive [21]. The effect of NaCl was described as follows: lower NaCl concentration reduces HOCl production, which is directly proportional to antibacterial efficacy and inversely proportional to corrosion and biologic compatibility [4].
By electrolysis of NaCl aqueous solution in a single cell electrolyzer, this research project aims to produce a safe disinfectant and sanitizer HOCl. This study project’s scope was divided into two parts. The first part focuses on laboratory work that examined at significant factors that affect the electrolysis of NaCl aqueous solution to produce HOCl. Using a UV-vis spectrophotometer, the second part focused on HOCl testing and analysis to determine the effectiveness in killing bacteria.
2. Methodology
2.1 Equipment and materials
The electrolysis unit, which included a DC power source, beaker, three types of electrodes (carbon, titanium, and graphite), ammeter and wires to complete the circuit, analytical balance, and stirrer, was the main equipment used in this research project. Ordinary table salt (NaCl), tap water, vinegar, pH paper, and an active chlorine test strip were used in this research study to conduct the electrolysis procedure.
2.2 Studied parameters
Starting with 0.5 grams of mass, the amount of NaCl was weighed using an analytical balance. The electrolyte was then fully dissolved in 1 liter of tap water or in a beaker. In the electrolyte, a selected carbon electrode was inserted. An ammeter was added to the circuit to measure the electric current during the electrolysis. During the electrolysis, a 12 V DC current was applied for 12, 24, 36, 48, and 60 minutes. Using pH paper and an active chlorine test strip, the concentration of HOCl and pH were determined every 12 minutes. The experiment was repeated with 5, 10, 15, 20, and 30 grams of NaCl on the same carbon electrode to determine the mass that yielded the highest concentration of HOCl After that, the same approach was used to test the selected mass of NaCl for another two electrodes, titanium, and graphite.
2.3 Bacteria growth test
Six samples were made by combining various amounts of HOCl with drain water and bacteria food to see if there was a significant variation in metal concentrations. For the six samples, the amount of HOCI added was 0, 2.5, 5, 7.5, 10, and 12 ml, with a constant volume of 2.5 ml drain water and 2.5 ml bacteria food. For bacteria growth, the samples were incubated at 35°C for 24 hours. All of the samples were tested for transmittance using a Shimadzu uv-vis spectrophotometer at a wavelength of 600 nm after 24 hours. The distilled water and bacteria food served as a blank for the UV-vis spectrophotometer measurements. The Optical Density 600 (OD 600) method was used to measure bacteria growth since the wavelength of 600 nm causes the least amount of damage to bacteria development. Because of the reflection of a dense bacteria population, bacteria growth increased as transmittance increased.
3. Results and discussion
3.1 The effect of Sodium Chloride (NaCl) amount on HOCl production during electrolysis
To determine the effect of sodium chloride quantity on HOCl concentration during electrolysis, a series of experiments were carried out. The reference base for the sanitizer concentration range is 50 ppm to 200 ppm, whereas the disinfection concentration range is 500 ppm to 800 ppm, according to the active chlorine test strip used. Table 1 shows the results of electrolysis of HOCl concentration, pH, and current with a carbon type electrode in 1 liter of tap water at different NaCl mass concentrations.
Since the NaCl concentration was so low, no HOCl was generated during the electrolysis for 0.5 grams of NaCl. Because of the slight difference in standard oxidation potentials, H2O is favored to be oxidized at the anode to produce O2. H2O’s standard oxidation potential is -1.36 V, while Cl-‘s standard oxidation potential is -1.23 V. Meanwhile, after 60 minutes of electrolysis, the concentration of HOCl measured by a chlorine test strip ranged from 25 to 50 ppm, which was sufficient for the oxidation of Cl- into free Cl2 at the anode to generate HOCl sanitizer with a minimum concentration of 50 ppm. The concentration of HOCl at 10 g of NaCl was initially 50 ppm at 12 minutes till 24 minutes, then increased to 200 ppm after 36 minutes, indicating that it was suitable for sanitizing. According to recent studies, pure non-iodized table salt was mixed with distilled water at a rate of 2 grams per liter to form a 0.2% salt solution, to which 5 milliliters of distilled white vinegar were added to produce HOCl in the 100ppm range [1]. Lower NaCl concentrations will minimize the amount of HOCl produced [4]
The concentration of HOCl increased when the amount of NaCl used was increased. After increasing the amount of NaCl to 15 grams, the concentration of HOCl reached 200 ppm after only 12 minutes of electrolysis and increased to 500 ppm after 36 minutes, which was the minimum concentration for disinfection application. For 20 grams of NaCl, the HOCl concentration increased from 200 ppm to 500 ppm after 24 minutes of electrolysis, whereas for 30 grams of NaCl, the concentration reached 500 ppm after 12 minutes of electrolysis and increased to 800 ppm after 36 minutes of electrolysis. Previous studies have shown that EW with HOCl concentrations as low as 100 ppm is effective against SARS-CoV-2 [4,19, 22], and various HOCl-based products that meet the EPA standards for use against SARS CoV-2 have been produced.
As a result, 10 g of NaCl can be determined as a sufficient amount to produce HOCl as a safe sanitizer in as little as 12 minutes, while 30 g of NaCl can be determined as a suitable amount to make HOCl as a safe disinfectant in as little as 12 minutes. Throughout the electrolysis process, the pH of the HOCl solution was slightly acidic in the range of 4 to 7, shifting the balance toward the more effective antibacterial HOCl [23].
3.2 The effect of electrode type on HOCl production during electrolysis
The effect of electrode type on HOCl production experiment was done on 10 and 30 grams of NaCl because it produced HOCl within the range of sanitizer and disinfectant concentrations. In sodium chloride solution electrolysis, the carbon electrode can produce HOCl. Carbon electrodes are used in electrolysis because of their ability to transmit electricity efficiently, their high melting point, and the large number of free electrons available for transfer, which can be used to facilitate various reactions. The carbon electrode, on the other side, gradually disintegrates in the solution, becoming thinner and eventually breaking into two pieces. Figure 3 shows how the solution becomes cloudy with carbon residue at the bottom of the electrolysis cell. Although the carbon electrode was able to produce HOCl, the disintegration issue must be addressed in the long run.
In HOCl electrolysis, the effect of using a titanium coated electrode was also tested. The electrolyte solution turns yellow during electrolysis, as illustrated in Figure 3, and no HOCl was formed when tested with an active chlorine test strip. Because the electrodes were dissolved in the electrolyte solution, the pH increased to 12, which was extremely alkaline. Because 100% pure titanium coated electrodes are extremely expensive, it was suspected that the titanium coated electrodes used were not pure enough. As a result, titanium coated electrodes were not recommended because the aim was to construct an electrolyzer at a low cost so that anyone could own one for personal use.
Graphite electrodes have been shown to produce clear HOCl solutions, are free of contaminants, and can produce the same concentration of HOCl as carbon electrodes. Due to its inexpensive cost, strong electrical and thermal conductivity, relatively inert behaviour in alkaline solution compared to metals, and porous structure with high purity, graphite is a good choice in water electrolysis compared to many other electrodes [21]. However, after a longer period of time, the pH of the solution increased to an alkali level, which is unfavorable for HOCl electrolysis. 1 mL of vinegar, a safe organic acid, was added to the solution to lower the pH to 5 to 7. The use of vinegar in a diluted salt solution to develop on-site EW at a dentist office using a portable EW generator unit was detailed in a recent study [1]. As a result, graphite was chosen as the best electrode because it produced a clear HOCl solution throughout the electrolysis process, as shown in Figure 3, and vinegar could be used as a safe and inexpensive raw material to lower the alkalic pH of the solution to pH 5–7, ensuring the presence of HOCl as an electrolysis product.
Figure 3. Visual observation comparison between carbon, titanium and graphite electrode
3.3 The effect of HOCl in reducing bacteria growth
In the turbidimetric method, a dense population of bacteria in a sample cuvette will reflect the light that passes through the sample cuvette in a UV-vis spectrophotometer. In other words, the intensity of the original light passing through the sample cuvette will reduce proportionally with the density of bacteria. Thus, if the transmittance is 1, which means the intensity of incoming light is equal to the intensity of light after passing through the sample cuvette, it is an indication of no bacteria growth. If the value of transmittance is near to zero, it means that the bacteria count in the sample is very dense. Table 2 illustrates the transmittance of 6 samples containing 5 mL bacteria solution with bacteria food and 500 ppm HOCl in a varying volume of 0, 2.5, 5, 7.5, 10.0, and 12.5 mL HOCl solution in each sample. All of the samples were incubated at 35°C for 24 hours to allow bacteria to grow before being tested for transmittance in a UV-vis spectrophotometer.
The transmittance measured after 24 hours of incubation when no HOCl solution was added was 0.194, indicating that the bacteria population was growing. The sample was cloudy due to the presence of a high bacteria population, as detected by thorough observation. The following sample consisted of 2.5 mL of HOCl solution. The measured transmittance was increased slightly to 0.363. In comparison to the first sample, this value indicates that the bacteria population was less dense. According to Table 2, the measured transmittance trend increased as the amount of HOCl solution increased from 0 to 12.5 ml, indicating that the population of bacteria decreased as the HOCl volume was raised. The transmittance was 1.0 at 12.5 ml HOCl, indicating that there were no microorganisms in the sample. This simple but successful procedure demonstrated that HOCl can inhibit bacterial growth.
4. Conclusions
The effects of the amount of NaCl and the type of electrodes on the electrolysis of NaCl aqueous solution to produce HOCl were studied in this research project. The results show that 10 grams of NaCl produces 50-200 ppm of HOCl, which is good for sanitizing, and 30 grams of NaCl produces 500-800 ppm of HOCl, which is good for disinfecting. Graphite was chosen as the best electrode because it has been shown to create clear HOCl solutions. The HOCl generated was proven to kill or reduce bacteria growth by using a simple transmittance method.
Acknowledgement
The researcher grateful and would like to express sincerely to University College TATI for supporting this research project.
References
[1] Farah R I and Ali S N A 2021 “Electrolyzed Water Generated On-Site as a Promising Disinfectant in the Dental Office During Covid-19” Pandemic Frontiers in Public Health 9:629142
[2] Hricova D, Stephan R and Zweifel C 2008 “Electrolyzed water and its application in the food industry” Journal of Food Protection 71:9 pp. 1934–1947
[3] Huang Y, Hung Y, Hsu S, Huang Y and Hwang D 2008 “Application of electrolyzed water in the food industry” Food Control 19 pp. 329-345
[4] Takeda Y, Uchiumi H, Matsuda S and Ogawa H 2020 “Acidic electrolyzed waterpotently inactivates SARS-CoV-2 depending on the amount of free available chlorine contacting with the virus” Biochem Biophys Res Commun. 530 pp. 1-3
[5] Yan P, Daliri Eb and Oh Dh 2021 “New clinical applications of electrolyzed water:a review” Microorganisms 9:136 doi:10.3390/microorganisms9010136
[6] Al-Haq M M, Sugiyama L J and Isobe S 2005 “Applications of electrolyzed water in agriculture and food industries” Food Sci. Technol. Res. 11:2 pp.135–150
[7] Wiant C 2013 “The chlorine residual: A public health safeguard, Water Quality and Health Council”
[8] Eryilmaz M and Palabiyik I M 2013 “Hypochlorous acid-analytical methods and antimicrobial activity” Tropical J. Pharmaceutical Research 12:1 pp. 123-126.
[9] Iram A, Wang X and Demirci Ali 2021 “Electrolyzed oxiding water and its application as sanitation and cleaning agent” Food Engineering Review 13 pp. 411-427
[10] Su Y C, Liu C and Hung Y C 2007 “Electrolyzed Water: Principles and Applications, in New Biocides Development, the combined approach of chemistry and microbiology” Peter Zhu ed. American Chemical Society Washington DC pp. 309-321
[11] Khalid N I, Ab Aziz N, Thani N M, Shapi’i R and Rahman N F A 2020 “Electrolyzed water as a sustainable cleaning and disinfection chemical for SMEs Malaysian meat processing food industries: Challenges and uncertainties” Journal of AgriculturalFood Engineering 1(1) 0006
[12] Shiroodi S and Ovissipour M 2018 “Electrolyzed water application in fresh produce sanitation” San Diego: Academic Press (Elsevier) pp.67-68
[13] Mourad K A and Hobro S 2020 “Deleveloping chlorine-based antiseptic by electrolysis” Science of The Total Environement 709 136108 ISSN 0048-9697
[14] Deza M A, Araujo M and Garrido M J 2003″ Inactivation of Escherichia coli O157:H7, Salmonella enteritidis and Listeria monocytogenes on the surface of tomatoes by neutral electrolyzed water” Letters of Applied Microbiology 37 pp. 482–487
[15] Deza M A, Araujo M and Garrido M J 2007 “Efficacy of neutral electrolyzed water to inactivate Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa and Staphylococcus aureus on plastic and wooden kitchen cutting boards” Journal of Food Protection 70:1 pp. 102–805 108
[16] Jun S Y, Kim Y H, Sung, J M , Jeong J W, Moon K D, Kwon J H and Lee Y K 2010 “Effects of Seed Decontamination Treatments on Germination of Red Radish Seeds during Presoaking” J Korean Soc Food Sci Nutr 39:10 pp. 1528-1534
[17] Al-Haq M, Seo Y, Oshita S and Kawagoe Y 2002 “Disinfection effects of electrolyzed oxidizing water on suppressing fruit rot of pear caused by Botryosphaeria berengeriana” Food Research International 35 pp. 657–664
[18] Song Y G and Lee S H 2019 “Efficacy of newly developed denture cleaning device on physical properties of denture material and Candida biofilm” J Dent Sci. 14:2 pp 48–54. doi: 10.1016/j.jds.2019.01.011
[19] Block M S and Rowan B G 2020 “Hypochlorous acid: a review” J Oral Maxillofac Surg. 78:146 pp 1–6. doi: 10.1016/j.joms.2020.06.029
[20] Khalid N I, Sulaiman N S and Ab Aziz N 2020 “Optimization of electrolysis parameters for green sanitation chemicals production using response surface methodology” Processes
[21] Yuvaraj A L and Santhanaraj D 2014 “A systematic study on electrolytic production of hydrogen gas by using graphite as electrode” Material Research 17(1) pp 83-87
[22] Sarada B V, Vijay R, Johnson R, Rao T N and Padmanabham G 2020 “Fight against COVID-19: ARCI’s technologies for disinfection” Trans Indian Natl Acad Eng 1–6. doi: 10.1007/s41403- 020-00153-3
[23] Pal P 2017 “Industrial Water Treatment Process Technology” Cambridge: Elsevier Science, Butterworth-Heinemann pp 43-4

